FUNCTION:
Skin lightening
DEFINITION:
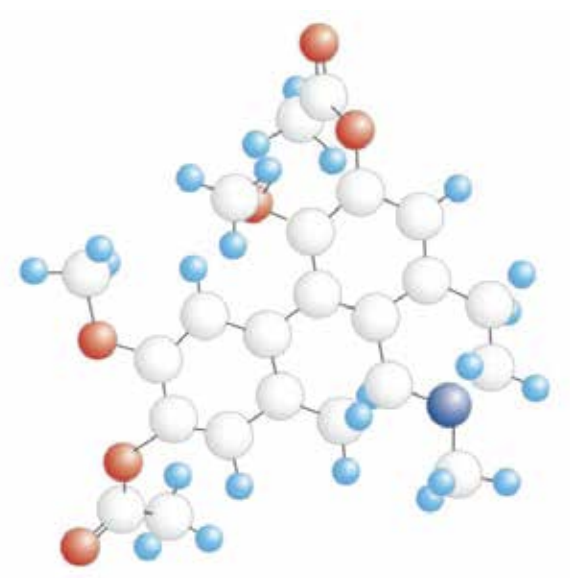
Diacetyl Boldine (DAB) in C C triglycerides.
PROPERTIES:
Diacetyl Boldine (DAB) works via α-adrenergic antagonist receptors and
calcium flow regulation.
POINT OF INTEREST:
Tyrosinase is not directly inhibited by competition, tyrosinase is stabilised in its
inactive form.
INCI NAME:
Caprylic/Capric Triglyceride – Diacetyl Boldine.
APPLICATIONS:
Emulsions, soaps, make-up products with lightening or whitening properties.
IN VITRO TESTS
TEST ON MELANOCYTE B16 LINE
TEST ON HUMAN MELANOCYTES
EX VIVO TEST
SKIN ETHIC® 3D MODEL
OF EPIDERMIS TYPE VI
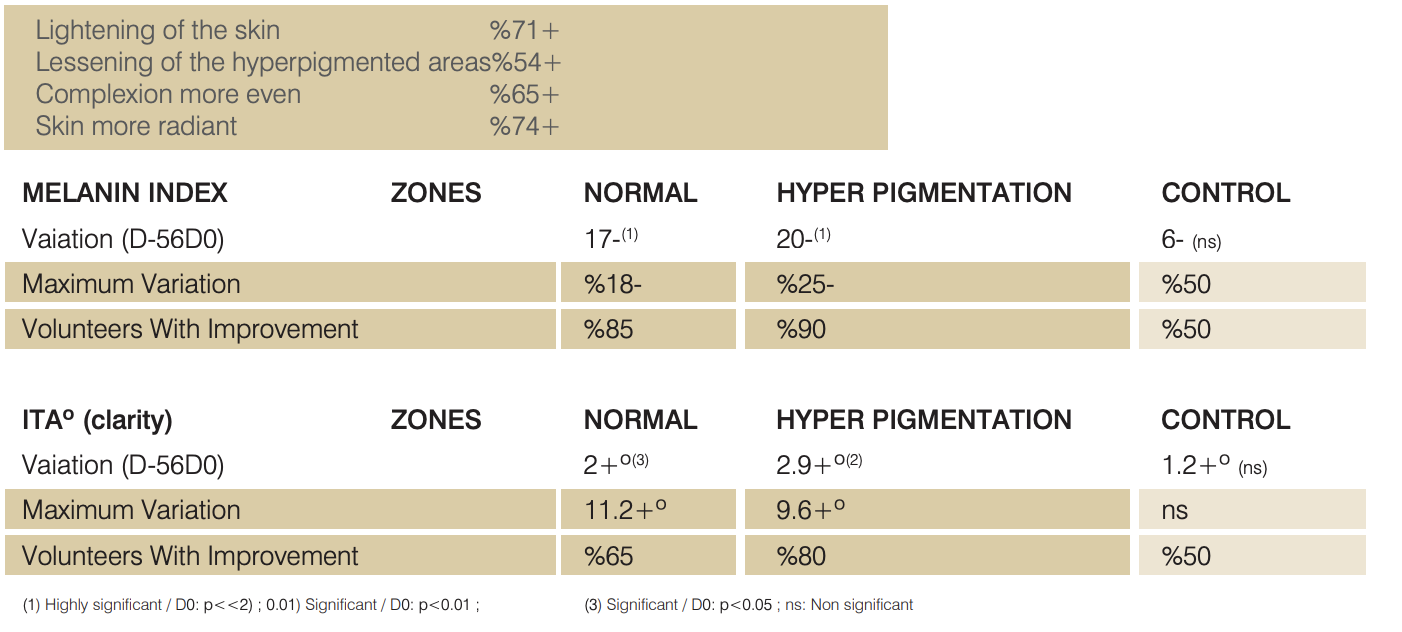
N VIVO TESTS ON ASIAN VOLUNTEERS
COLORIMETRIC TEST
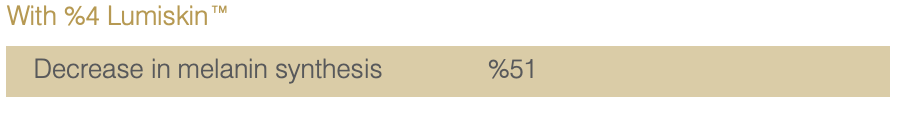
Study on 20 Asian Volunteers.
Twice daily application of a cream containing %4 Lumiskin™ (40 ppm DAB) for 2 months.
Measurement of ITA° (clarity) by chromameter and Melanin Index by mexameter on one homogeneous area (two sites),
one hyperpigmeted area and one untreated zone (chin).
SELF-EVALUATION